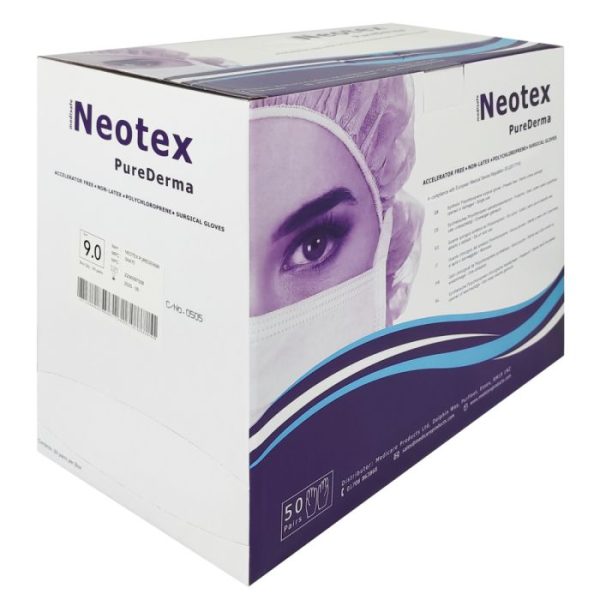
Neotex PureDerma Polychloroprene Accelerator‑Free Surgical Gloves – SNA
Neotex PureDerma Polychloroprene Accelerator‑Free Surgical Gloves have been developed to reduce the risk of latex-related Type I Hypersensitivities and the Type IV Hypersensitivities caused by the chemical accelerators used in the manufacture of medical gloves. These gloves are free from latex and chemical accelerators. Manufactured on ergonomically designed formers from an advanced synthetic formulation, the Neotex PureDerma offers ultimate comfort and tactile sensitivity.
Product Colour: Cream
Packing Contents – All: 1 pair per pouch, 50 gloves per dispenser, 4 dispensers per case, 30 cases per pallet
Size Range: 5.5-9
Fingertip Thickness: 0.2 mm
Palm Thickness: 0.18 mm
Typical Weight: 14 g
- Description
Description
Product Highlights
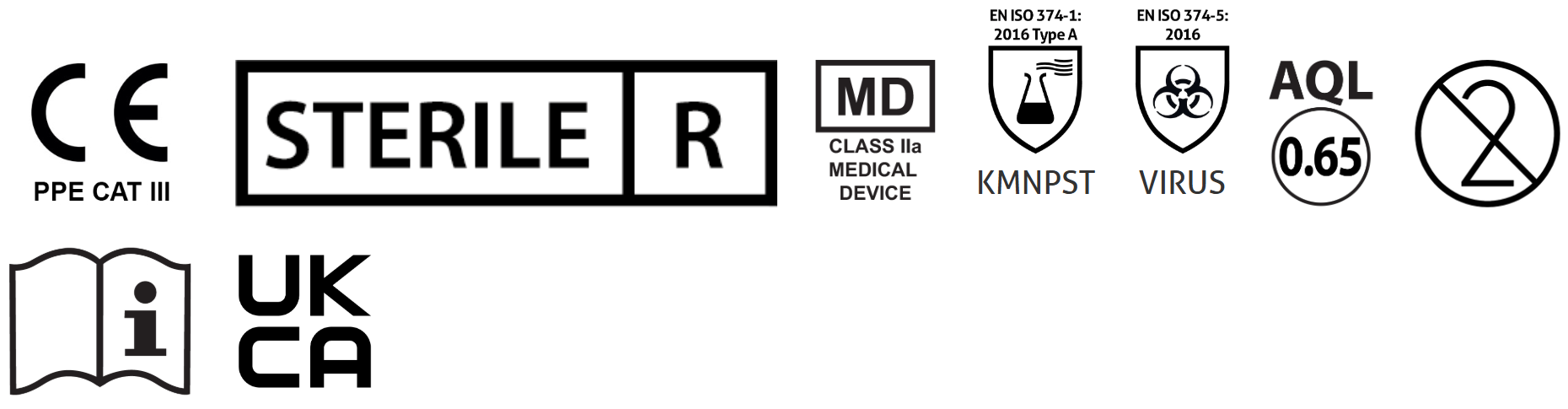
- Sterilised by irradiation method
- Accelerator free synthetic gloves removing the risk of contact dermatitis caused by these chemicals
- Micro Roughened to provide excellent feel and instrument grip in both wet and dry conditions
Comfort:
- Manufactured on ergonomically designed formers from an advanced synthetic formulation, offers ultimate comfort and tactile sensitivity
Fit:
- Hand specific to reduce hand fatigue
Sensitivity:
- Latex free, reducing the risk of hypersensitivity and allergic reactions
Disposable Gloves Standards:
- AQL 0.65
- Compliant with European Standards EN455: Medical Gloves for Single Use parts 1,2,3&4
- Manufactured in accordance with Personal Protective Equipment (PPE) by the European PPE Regulation (EU) 2016/425
- Tested to European Standards EN420 & EN ISO 374-1 & EN ISO 374-5
- This product is classified as Class IIa Medical Device in compliance with the requirements of Medical Directive 93/42/EEC on Medical Devices (MDD)
Related Products
Tailored Industrial Support with Quality Products and Expert Guidance.
Our solutions will reduce your bottle necks and improve your efficiency and productivity, contact us now and let us know how we can help you, we are always here!